Contents
Nucleic Acids & Inorganic Ions
Inorganic Ions
Inorganic ions occur in the cytoplasm of organisms in both high and low concentrations. Each type of ion has a specific role that is relevant to a range of topics across A-Level. The key ions that you need to be familiar with are listed below:
- Hydrogen ions and pH – this is relevant when considering enzymes and proteins denaturing (topic 1), increasing heart rate (topic 6), and the Bohr effect on hemoglobin (topic 3).
- Iron ions as a component of hemoglobin – this links to quaternary structure proteins (topic 1) and the function of hemoglobin (topic 3).
- Sodium ions in the co-transport of glucose and amino acids – this is relevant in the absorption of glucose (topic 3) and resting/action potential in the nervous systems (topic 3).
- Phosphate ions as components of DNA and of ATP – this is relevant to DNA, RNA, and ATP structure (topic 1).
Nucleic Acids
ATP
ATP contains three phosphate ions that play a significant role in energy transfer. It is essential to metabolism, which is all the chemical reactions that take place in a cell. ATP, or Adenosine Triphosphate, is an immediate source of energy for biological processes. Metabolic reactions in cells require a constant, steady supply of ATP.
The emphasis is on IMMEDIATE energy source. This is what is unique about ATP compared to other molecules that release energy, such as glucose, and therefore must be stated in exams to get the mark.
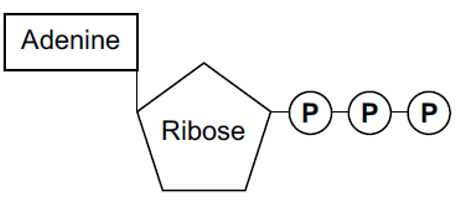
Below is a diagram of ATP, and this is the level of detail that you need to remember the structure in. ATP is comprised of adenine, a nitrogenous base (meaning a base that contains nitrogen), ribose (a pentose sugar), and three inorganic phosphate groups. The phosphate groups are described as being inorganic because they do not contain any carbon atoms. For this reason, in chemical reactions, the symbol to represent this is a P for phosphate and i for inorganic -Pi.
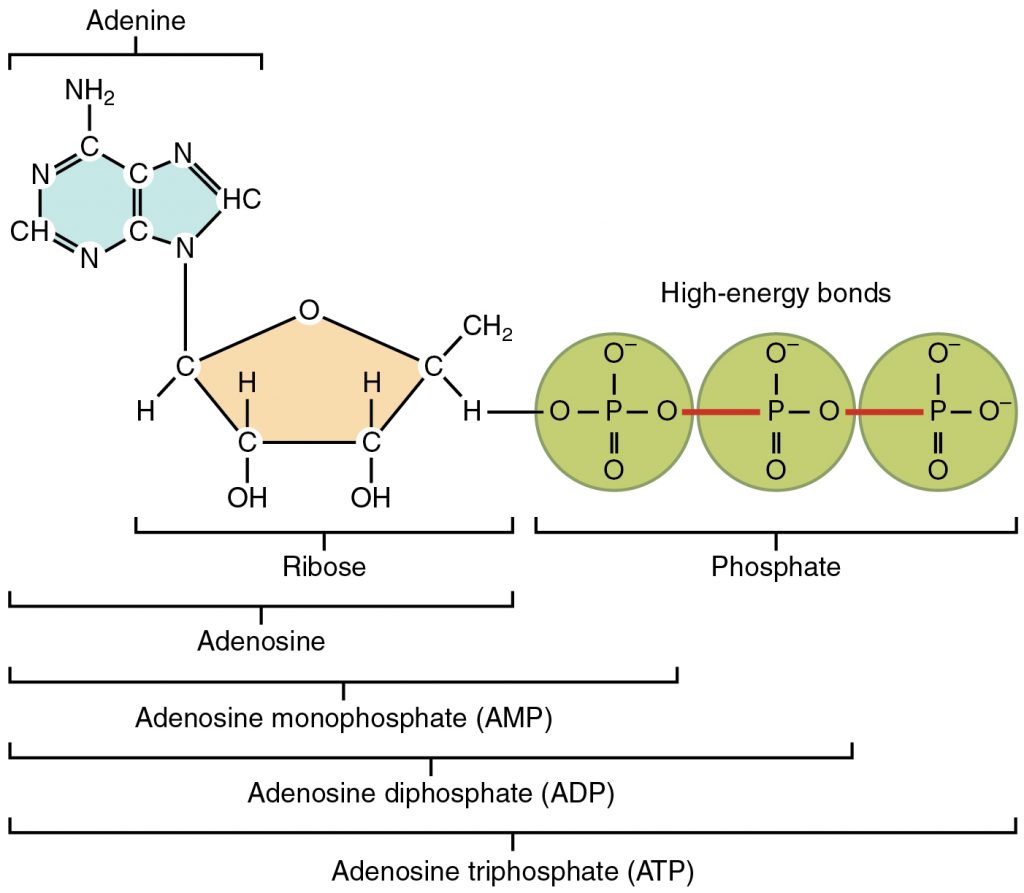
A more detailed diagram of ATP is below:
ATP is made during respiration. ATP is made from ADP, adenosine diphosphate, by the addition of an inorganic phosphate via a condensation reaction and using the enzyme ATP synthase. ATP can be broken down, or hydrolyzed, into ADP + Pi by a hydrolysis reaction and the enzyme ATP hydrolase.
ATP + H2O –> ADP + Pi
The bonds between the inorganic phosphate groups are high energy bonds, as shown in the diagram above. Therefore, by breaking one of these bonds, a small amount of energy is released to the surroundings, which can be used in chemical reactions. This is why ATP is an immediate energy source- only one bond has to be hydrolyzed to release energy, and as ATP cannot be stored, this occurs straight away. It is essential to remember that ATP cannot be stored. ATP is not only able to release energy to the surroundings, but it can also transfer energy onto different compounds. The inorganic phosphate released during the hydrolysis of ATP can be bonded onto completely different compounds to make them more reactive. This is known as phosphorylation, and this actually happens to glucose at the start of respiration to make it more reactive.
ATP Properties
There are five key properties that ATP has, making it a suitable immediate source of energy. In exam questions, ATP properties are frequently compared to glucose to emphasize why ATP is the immediate source of energy for cells rather than glucose. This is explained and demonstrated in the five points below:
- ATP releases energy in small, manageable amounts so no energy is wasted. This means that cells do not overheat from wasted heat energy, and cells are less likely to run out of resources. In comparison to glucose, this would release large amounts of energy that could result in wasted energy.
- It is small and soluble to be easily transported around the cell. ATP can move around the cytoplasm with ease to provide energy for chemical reactions within the cell. This is a property ATP has in common with glucose.
- Only one bond is broken/hydrolyzed to release energy, which is why energy release is immediate. Glucose would need several bonds to be broken down to release all its energy.
- It can transfer energy to another molecule by transferring one of its phosphate groups. ATP can enable phosphorylation, making other compounds more reactive. Glucose cannot do this, as it does not contain phosphate groups.
- ATP can’t pass out of the cell. The cell always has an immediate supply of energy. ATP cannot leave the cell, whereas glucose can. This means that all cells have a constant supply of ATP or ADP + Pi, but a cell can run out of glucose.
- What is the function of ATP?
- Your answer should include: Immediate / Source / Energy