Contents
Enzymes
Structure & Function
What are some of the key terms associated with enzymes?
- Active site
- Protein
- Enzyme-substrate complex
- Biological catalyst
Enzymes are biological catalysts made of proteins. They speed up reactions without being used up themselves. Some of the essential reactions they catalyze include respiration, photosynthesis, protein synthesis, and digestion. Having studied the structure and function of proteins, this theory can now be applied to enzymes. While enzymes are relatively large molecules, only a small part of the enzymes attaches to a substrate to catalyze a reaction. This site is known as the active site. The active site is specific and unique in shape due to the specific folding and bonding in the tertiary structure of the protein. Due to this specific active site, enzymes can only attach to substrates that are complementary in shape.
- Are enzymes globular or fibrous proteins?
- Globular
- What stage in protein development results in a unique 3D structure held in place by hydrogen, ionic, and disulfide bonds?
- Tertiary
Two Models of Enzyme Action
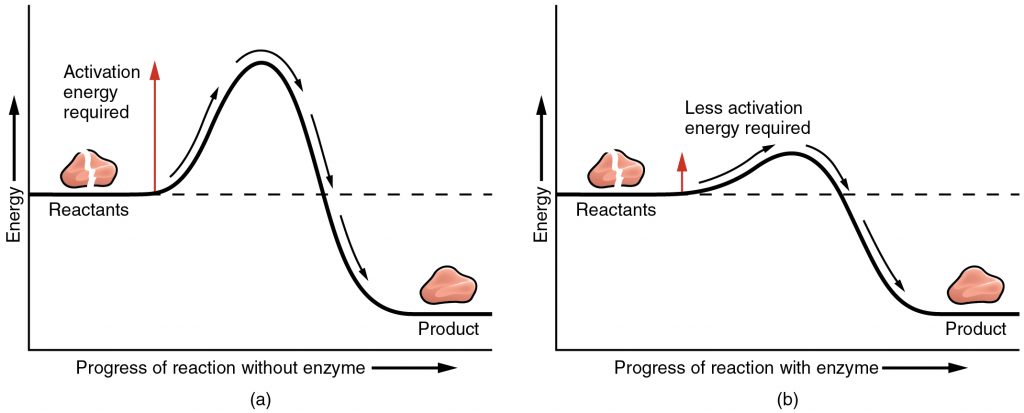
We have stated above the structure and function of enzymes, and next is to explain how enzymes speed up reactions by attaching to substrates. All reactions require a certain amount of energy before they occur (diagram a), this is known as the activation energy. When enzymes attach to the substrate, they can lower the activation energy needed for the reaction to occur (diagram b), and therefore speed up the reaction. There are two models to explain how this occurs.
Lock & Key Model
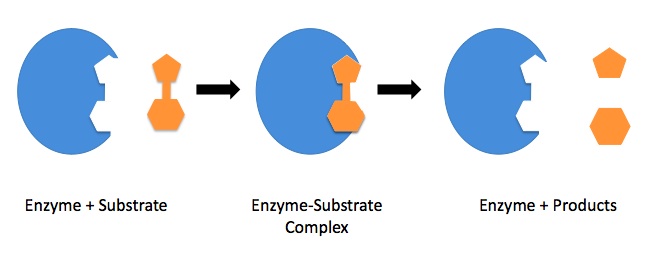
This model suggests that the enzyme is like a lock and that the substrate is like a key that fits into it due to their complementarity in shape. This model suggests that the enzyme active site is a fixed shape and that due to random collision, the substrate can collide and attach to the enzyme. This forms an enzyme-substrate complex.
The term enzyme-substrate complex is always a marking point for enzyme questions!
Once the enzyme-substrate complex has formed, the charged groups within the active site are thought to distort the substrate and therefore lower the activation energy. The products are then released, and the enzyme active site is empty and ready to be reused.
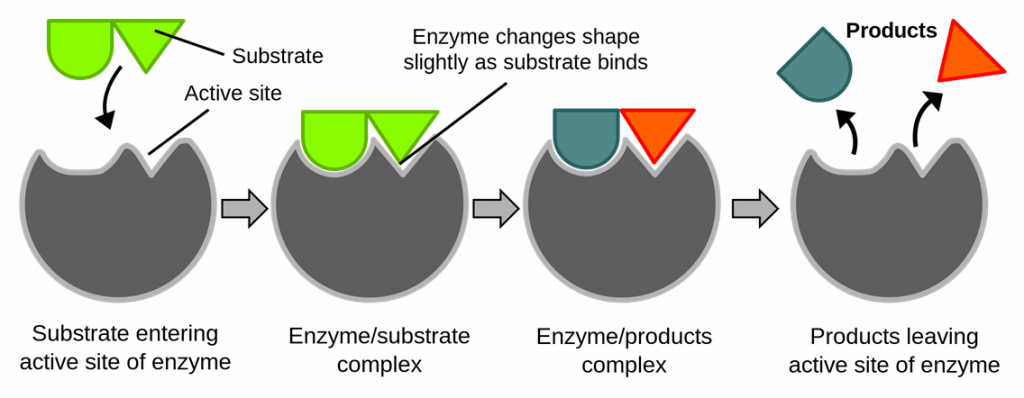
Induced Fit Model
This model suggests that the enzyme is like a glove and the substrate is like your hand; the empty glove is not exactly complementary in shape to your hand, but when your hand enters it enables the glove to mold around your hand to become completely complementary. This analogy describes the process well. The induced fit is when the enzyme active site is induced, or slightly changes shape, to mold around the substrate. When the enzyme-substrate complex occurs, due to the enzyme molding around the substrate, it puts strain on the bonds and therefore lowers the activation energy. The products are then removed, and the enzyme active site returns to its original shape. The induced fit model is the accepted model for how enzymes function.
In summary, what is the difference between the two models? The induced fit model suggests that the active site changes shape and then becomes complementary in shape, whereas the lock and key model suggests enzymes are a fixed shape, and the active site does not change.
- Gangliosides are lipids found on the cell surface of membranes of nerve cells. Hexosaminidase is an enzyme present in blood that breaks down gangliosides. If gangliosides are not broken down, they damage nerve cells. Hexosaminidase only breaks down gangliosides. It does not break down other lipids. Explain why this enzyme only breaks down gangliosides.
- Your answer should include: Active / Site / Fits
- Describe the induced fit model of enzyme action:
- Your answer should include: Not / Complementary
- Describe one way that the lock and key model is different from the induced fit model:
- Your answer should include: Active / Site / Fixed
Factors Affecting Enzymes
Enzymes, which are proteins, are sensitive to certain conditions:
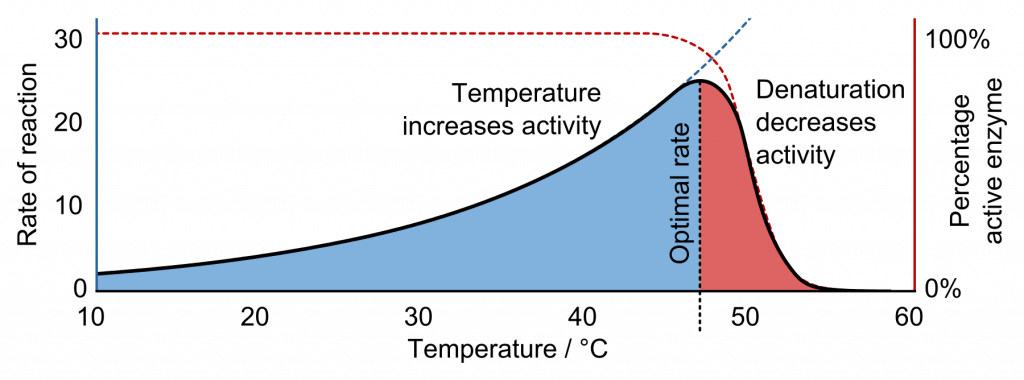
Temperature
If the temperature is too low, there is not enough kinetic energy for successful collisions. If the temperature is too high, enzymes denature, the active site changes shape, and enzyme-complexes cannot form.
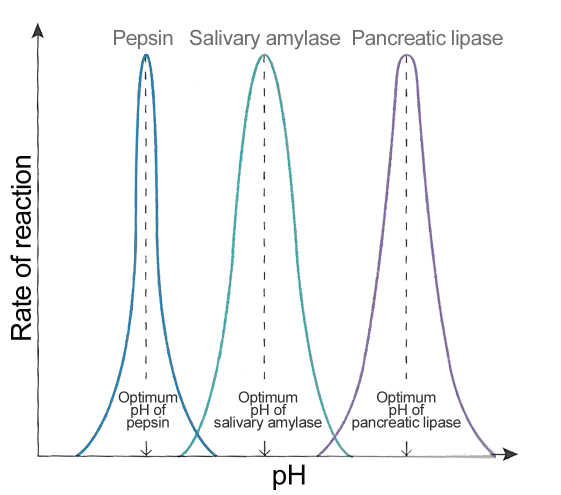
pH
Too high or too low a pH will interfere with the charges in the amino acids in the active site. Therefore the enzyme denatures.
Substrate and Enzyme Concentration
If there is not enough substrate added, then the reaction will be slower as there will be fewer collisions between the enzyme and substrate. If there are not enough enzymes, then the enzyme active sites will become saturated with substrate and unable to work any faster.
Enzyme Inhibitors
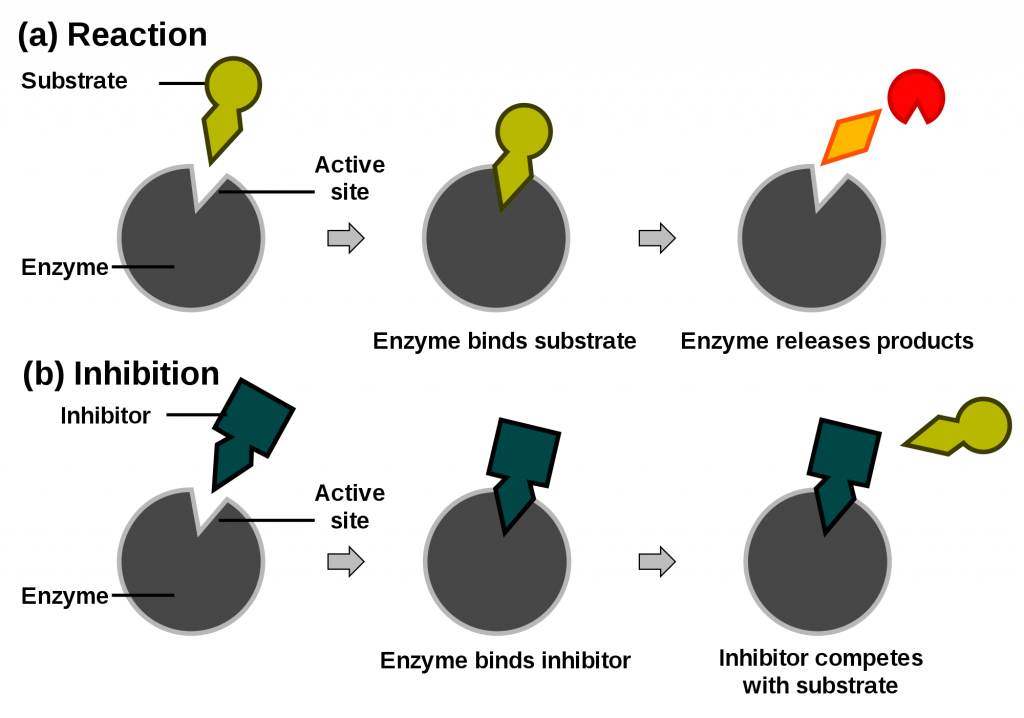
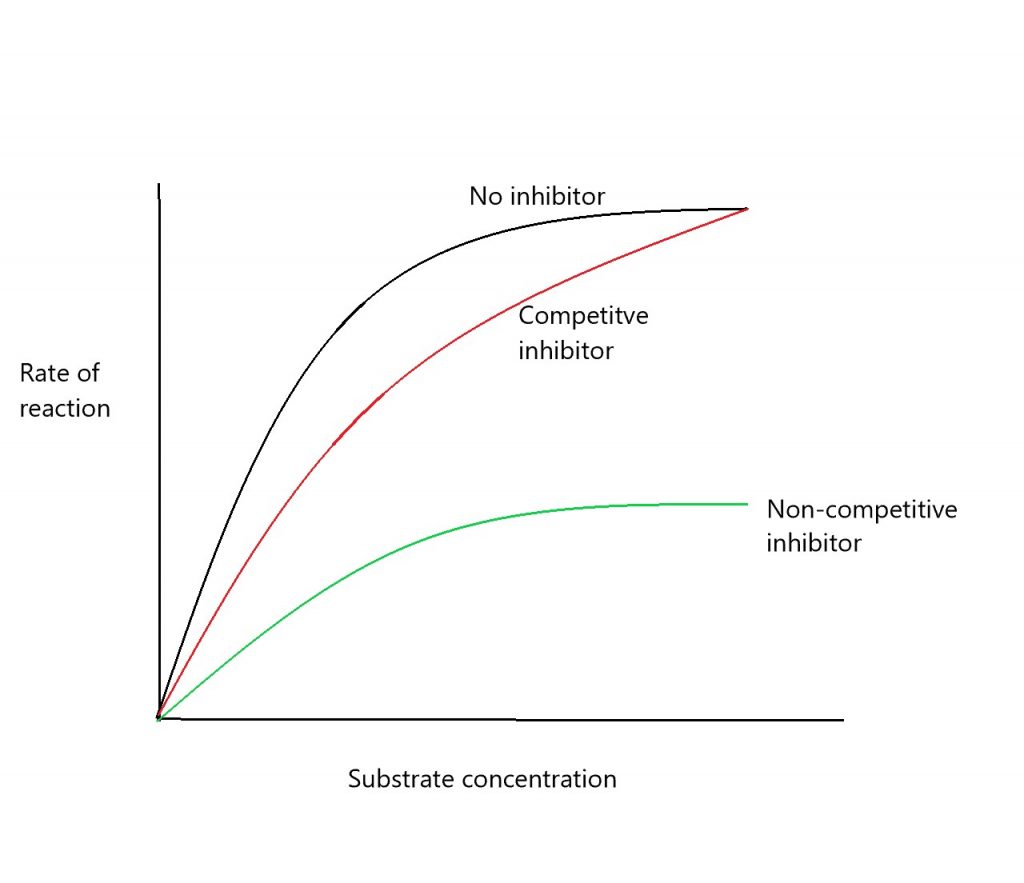
Competitive inhibitors are the same shape as the substrate and can bind to the active site. This prevents the substrate from binding, and the reaction occurring. If you add more substrate, this will flood/out-compete the inhibitor, knocking them out of the active site.
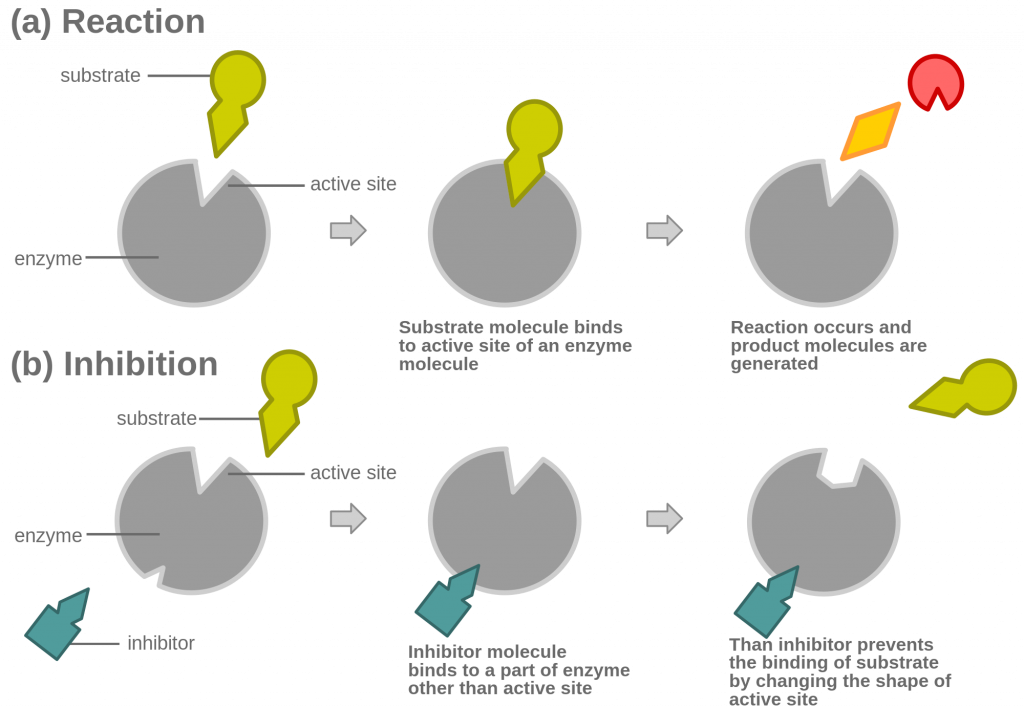
Non-competitive inhibitors bind to the enzyme away from the active site, the allosteric site. This causes the active site to change shape, and therefore the substrate can no longer bind, regardless of how much substrate is added (see graph below):